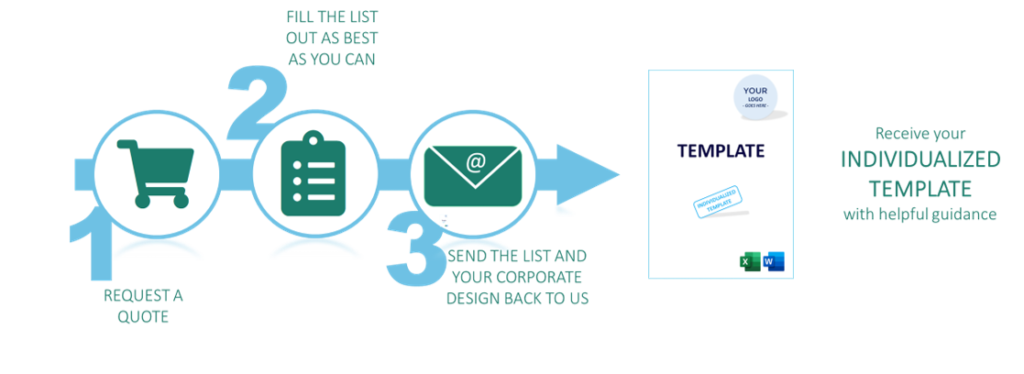
Use our templates full of helpful guidance to create your Technical Documentation or your QMS. Save even more time with pre-filled, individualized templates with your logo.
We review or create selected documents or complete technical documentationd of medical devices or IVD products. Thereby, we ensure that they are MDR or IVDR compliant.
Set up your QMS by using our templates, our input, our hands-on-help or let us do (nearly) all of the work. Whichever way you choose, we will get your QMS audit-ready.
Come to us with your questions. We offer answers and solutions for topics related to regulations, standards, QMS, strategic decisions, CE marking, or market access.
You want to conduct a performance study according to IVDR but your company is not established in the Union? We can take on the role as legal representative for you.
Many stakeholders are unsure about the quality and completeness of the Technical Documentation. Since the Technical Documentation is the regulatory heart of your medical device or IVD product (MDR/IVDR), you need to know about all the gaps. Our GAP analysis will give you a comprehensive overview. This will enable you to focus on the biggest issues and gaps.
This is not only important if you are a manufacturer and therefore in the possession of your own Technical Documentation. It might also be important, if you are an investor or EC representative and you are unsure, if the quality of the documentation of your partner company is sufficient.
Sometimes, however, a quick check might not be enough for your purposes. Instead, you may need a detailed MDR or IVDR compliance check. In this case, a document review performed by our experts might me a better option. During the review process, our experts will check if all requirements of MDR/IVDR regulations, from applicable harmonized standards, as well as acknowledged guidance documents are fulfilled. This will enable you to get your documentation Audit-ready quickly and efficiently.
You need Technical Documentation for your EU market launch? Whether individual documents or the entire Technical Documentation: our experts work quickly, reliably and ensure the highest quality. Audit-ready documents are created in close consultation with you.
You need to know if the QM system is audit-ready? In this case, we conduct ISO 13485 conform internal audits for you at your company or on your suppliers’ premises. Our qualified auditors prepare all the necessary documentation required for a successful audit: Audit plan, audit agenda and of course the audit report.
The selection of a Notified Body is an important step on your way to CE, especially in times of low audit and review capacities at Notified Bodies. We can support you in selecting a suitable Notified Body – taking into account your company-wide regulatory strategy. In addition, we can take over the communication with the Notified Body completely for you. Of course, the clarification of non-conformities with you is also part of our portfolio.
You are planning to conduct a performance study according to IVDR (EU) 2017/746 in one or more countries of the Union? In this case, you need a legal representative who is responsible for ensuring compliance with your obligations persuant to IVDR and who acts as a contact on your behalf (IVDR Article 58). AstraCon can take those responsibilities on. If needed, we can also support you in planning and executing your submission strategy.
Contact us
TEMPLATES
TECHNICAL DOCUMENTATION
MDR / IVDR
ISO 13485
Notified Buddy is here to help!
LEGAL REPRESENTATIVE